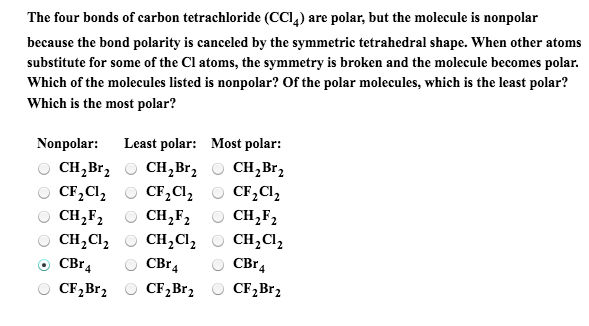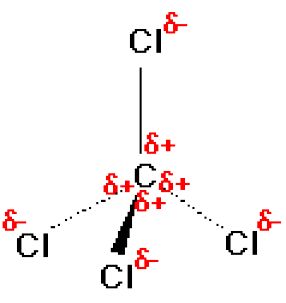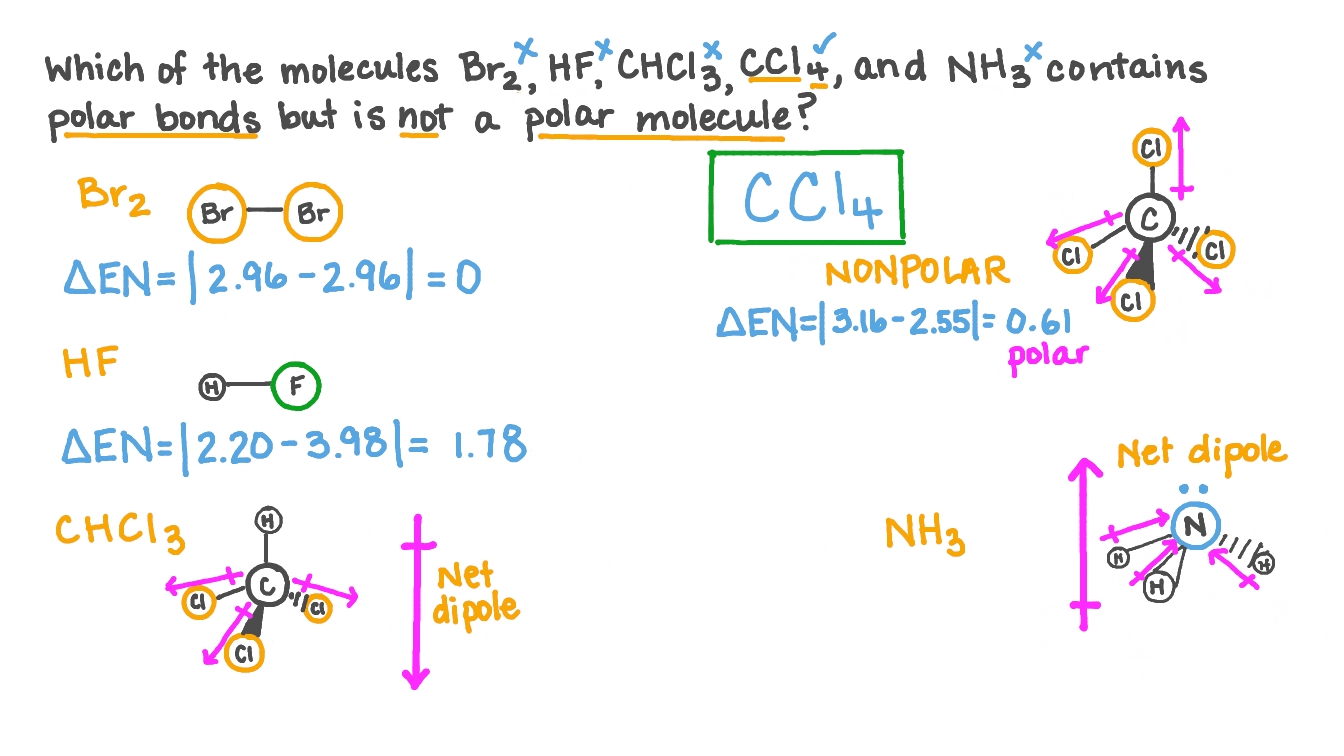
Question Video: Determining the Molecule That Contains Polar Bonds but Is Not a Polar Molecule | Nagwa

Which solvent, water or carbon tetrachloride, would you choose to dissolve each of the following? a. KrF_2 b. SF_2 c. SO_2 d. CO_2 e. MgF_2 f. CH_2O g. CH_2=CH_2 | Homework.Study.com

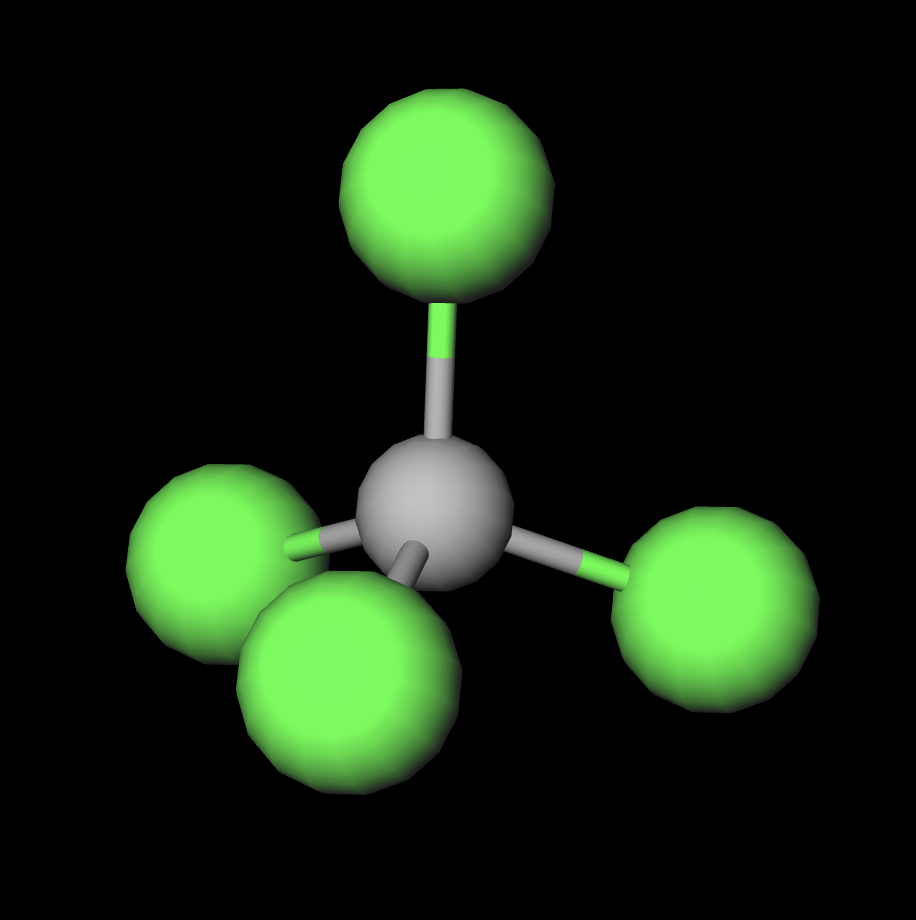
Carbon Tetrachloride (CCl4); Lewis Structure, Molecular Geometry, Polarity, And Applications | Scientific Sarkar

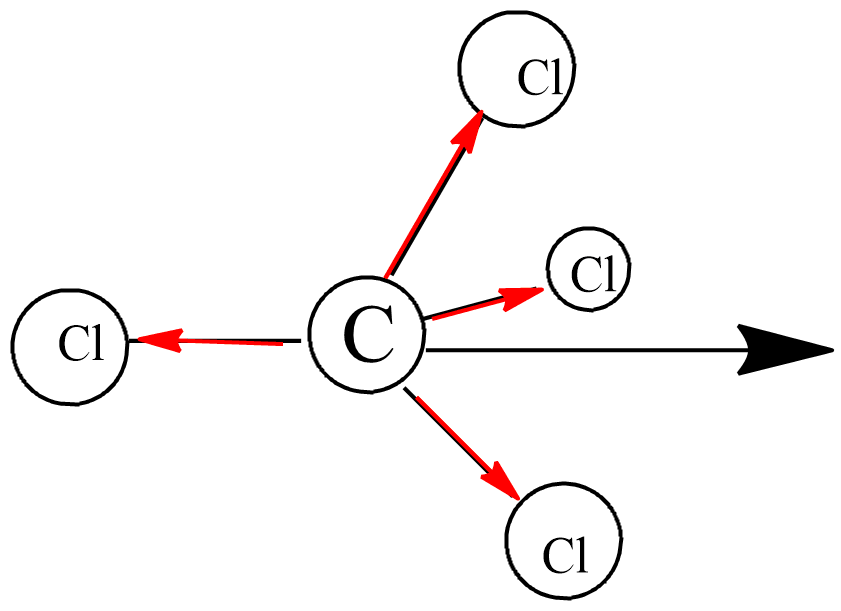
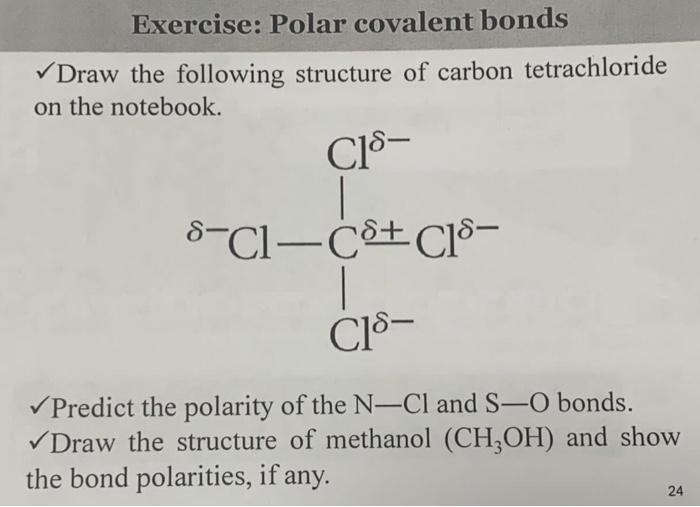
SOLVED: Explain how a molecule that contains polar bonds can be nonpolar. In your answer, use carbon tetrachloride, CCl4, as an example.

SOLVED: Explain how it is possible for CCl4 to have polar bonds but be a non-polar molecule. A diagram may be helpful in your answer, but it must be explained. The bonds

Not every molecule with polar bonds is polar. Explain this statement. Use CCl_4 as an example. | Homework.Study.com

Carbon tetrachloride CCl4 lewis dot structure, molecular geometry, polar or nonpolar, Bond angle | Molecular geometry, Molecular shapes, Molecular