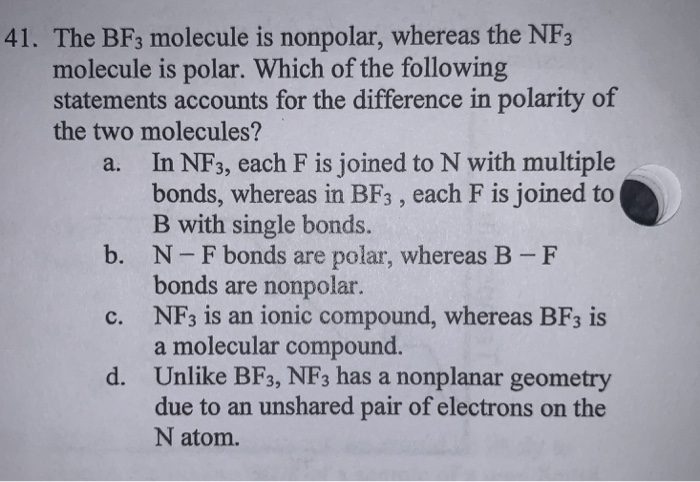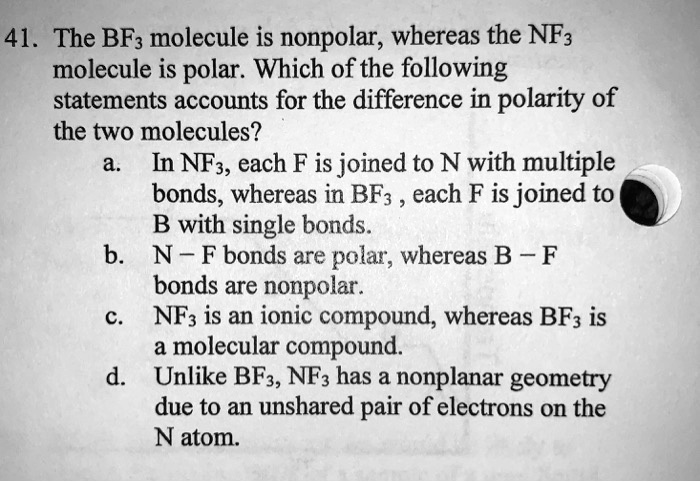
SOLVED: 41. The BF3 molecule is nonpolar; whereas the NF: molecule is polar: Which of the following statements accounts for the difference in polarity of the two molecules? In NFs, each F

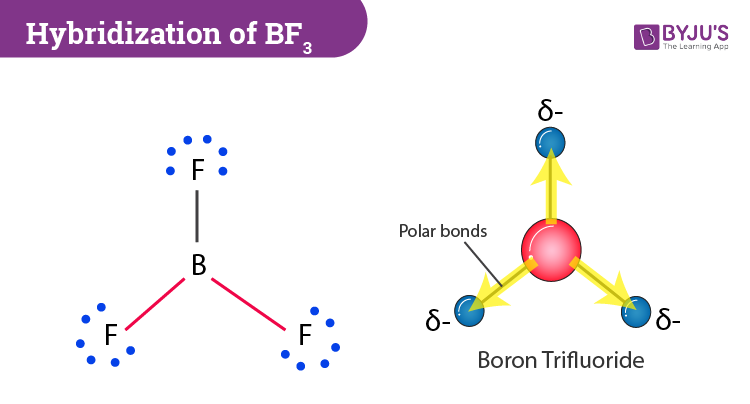
Boron Trifluoride BF3 is a non polar molecule whereas ammonia NH3is a polar molecule. The difference in polarity is related to the fact that

BF3 and NF3 both are covalent compounds but NF3 is polar whereas BF3 is non - polar. This is because :
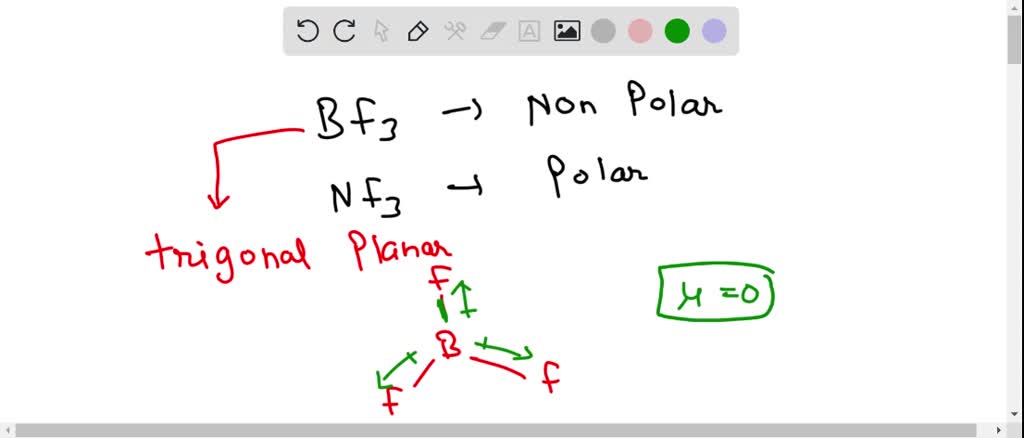
SOLVED: 41. The BF3 molecule is nonpolar; whereas the NF: molecule is polar: Which of the following statements accounts for the difference in polarity of the two molecules? In NFs, each F

The molecule BF3 and NF3 , both are covalent compounds but BF3 is non - polar and NF3 is polar. The reason is that: